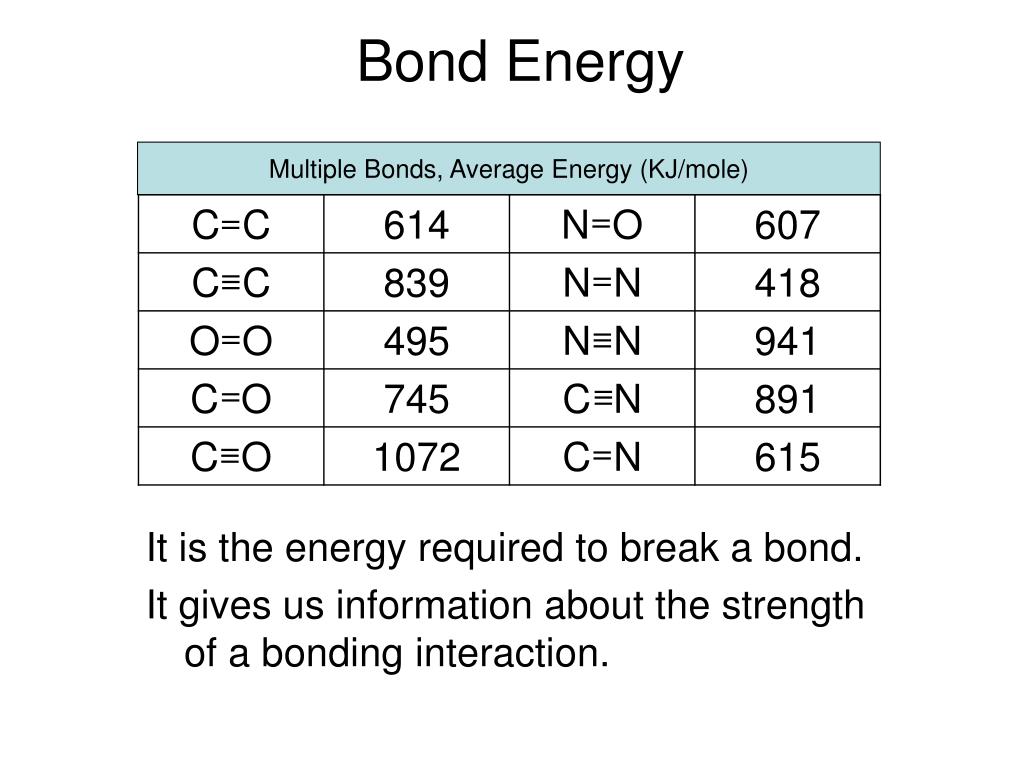
Example Of Bond Energy . Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Definition of hydrogen molecules bond energy. Web chemical bond energy example. Energy is released to generate bonds, which is why the enthalpy change for. To define and used average bond. Hydrogen molecules (h 2) have a single h h bond. This bond has an energy of. Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms.
from www.slideserve.com
Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. Hydrogen molecules (h 2) have a single h h bond. Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; This bond has an energy of. To define and used average bond. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Web chemical bond energy example. Energy is released to generate bonds, which is why the enthalpy change for. Definition of hydrogen molecules bond energy.
PPT Covalent Bonding PowerPoint Presentation, free download ID6711760
Example Of Bond Energy Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. To define and used average bond. Hydrogen molecules (h 2) have a single h h bond. This bond has an energy of. Energy is released to generate bonds, which is why the enthalpy change for. Web chemical bond energy example. Definition of hydrogen molecules bond energy. Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule;
From gy.kimiq.com
Table Of Bond Energies Energy Etfs Example Of Bond Energy Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Definition of hydrogen molecules bond energy. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Web chemical bond energy example. Web the bond energy is. Example Of Bond Energy.
From www.tes.com
6.4.3 Bond energies (AQA KS3 Activate 2) Teaching Resources Example Of Bond Energy In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. This bond has an energy of. Definition of hydrogen molecules bond energy. Hydrogen molecules (h 2) have a single h h bond. Web. Example Of Bond Energy.
From sciencenotes.org
Bond Energy and Strength Example Of Bond Energy Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; Hydrogen molecules (h 2) have a single h h bond. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Web the bond energy is a measure of the amount of. Example Of Bond Energy.
From pathwaystochemistry.com
Table of Bond Energies Pathways to Chemistry Example Of Bond Energy Web chemical bond energy example. Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Definition of hydrogen molecules bond energy. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently. Example Of Bond Energy.
From www.slideshare.net
Tang 06 bond energy Example Of Bond Energy Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. This bond has an energy of. Web chemical bond energy example. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Hydrogen molecules (h 2) have a single h h bond. Web putting. Example Of Bond Energy.
From quizconsectary.z21.web.core.windows.net
How To Calculate Bond Energy Example Of Bond Energy Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. This bond has an energy of. Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; To. Example Of Bond Energy.
From www.pinterest.com
Bond Energy Easy Science Chemistry experiments, Bond, Flashcards Example Of Bond Energy Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Hydrogen molecules (h 2) have a single h h bond. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded. Example Of Bond Energy.
From eduinput.com
Bond Energy, definition, types, factors and applications Example Of Bond Energy Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. Hydrogen molecules (h 2) have a single h h. Example Of Bond Energy.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Example Of Bond Energy To define and used average bond. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Definition of hydrogen molecules bond energy. Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Web the bond energy. Example Of Bond Energy.
From www.slideserve.com
PPT Unit 5 Bonding PowerPoint Presentation, free download ID2421152 Example Of Bond Energy Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; To define and used average bond. In the chemical bonds of a molecule the attractive electrical forces cause bound states to. Energy is released to generate bonds, which is why the enthalpy change for. Web putting. Example Of Bond Energy.
From pt.slideshare.net
Tang 06 bond energy Example Of Bond Energy Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Web chemical bond energy example. To define. Example Of Bond Energy.
From www.wikihow.com
How to Calculate Bond Energy 12 Steps (with Pictures) wikiHow Example Of Bond Energy Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. This bond has an energy of. To define and used average bond. Web the bond energy is a measure of the amount of energy needed to break apart one mole of. Example Of Bond Energy.
From www.slideserve.com
PPT Bond energy & activation energy PowerPoint Presentation, free Example Of Bond Energy This bond has an energy of. Web chemical bond energy example. Energy is released to generate bonds, which is why the enthalpy change for. To define and used average bond. Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. In. Example Of Bond Energy.
From www.slideserve.com
PPT Bond Energies PowerPoint Presentation, free download ID5741555 Example Of Bond Energy Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. Energy is released to generate bonds, which is why the enthalpy change for. This. Example Of Bond Energy.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID6711760 Example Of Bond Energy Web chemical bond energy example. Definition of hydrogen molecules bond energy. Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Energy is released to generate bonds, which is why the enthalpy change for. Web the bond energy is a measure. Example Of Bond Energy.
From www.slideshare.net
Tang 06 bond energy Example Of Bond Energy Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Definition of hydrogen molecules bond energy. Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; In. Example Of Bond Energy.
From www.tes.com
Bond Enthalpy OCR A level Chemistry Teaching Resources Example Of Bond Energy Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; Energy is released to generate bonds, which. Example Of Bond Energy.
From sciencewiz.com
Chemical Bonds Store Energy ScienceWiz Example Of Bond Energy Definition of hydrogen molecules bond energy. Web the exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; Web putting in +436 kj per mole of hydrogen molecules (the bond enthalpy) pushes the hydrogen into a higher energy state by breaking the bonds and making individual atoms. To. Example Of Bond Energy.